Restricted Agricultural Remedies
![]() Click here to refer to our FAQ if you have queries that might not be covered in the below sections.
Click here to refer to our FAQ if you have queries that might not be covered in the below sections.
On 25 August 2023, new regulations were published under the Fertilizer, Farm Feeds, Agricultural Remedies and Stock Remedies Act (Act No. 36 of 1947). Although this Act regulates various products and processes, these new regulations are specific to the products referred to as “agricultural remedies” and is known as the “Regulations relating to agricultural remedies”.
These regulations define a new class of chemicals referred to as “restricted agricultural remedies”.
According to the regulation, a “restricted agricultural remedy” is defined as an agricultural remedy for which the Registrar, out of concern for its human health or environmental risks, has set out additional information to be shown on the label concerning essential conditions in respect of the display, distribution or limitations on use of, or qualifications of persons who may use the agricultural remedy, and such remedy shall comply with the criteria as set out in Annexure A.
According to Annexure A (3), agricultural remedy formulations fulfil the restricted agricultural remedy criteria when such agricultural remedy has one or more of the following characteristics:
- Criterion 1: Agricultural remedy formulations that meet the criteria of classes Ia or Ib of the WHO Recommended Classification of Pesticides by Hazard or;
- Criterion 2: Agricultural remedy formulations that meet the criteria of acute toxicity categories 1 or 2 of the GHS;
- Criterion 3: Agricultural remedy active ingredients and formulations listed by the Rotterdam Convention in its Annex III; and
- Criterion 4: Agricultural remedy active ingredients and formulations that have shown a high incidence of severe or irreversible adverse effects on human health or the environment.
In layman’s terms:
- The agricultural remedies classified under Criterion 1 are restricted due to acute toxicity and were previously red band products (before the recent implementation of GHS). For more information on the WHO classification scheme and active ingredients affected, please click here.
- Similarly, agricultural remedies classified under Criterion 2 are restricted due to acute toxicity but may include additional remedies in comparison to criterion 1 as the GHS considers additional routes of exposure (inhalation), as well as the toxicity of co-formulants, which is not considered by the WHO classification scheme in all cases. For more information on GHS, please click here.
- The agricultural remedies restricted under Criterion 3 are listed in Annex III of the Rotterdam Convention and are chemicals that have been banned or severely restricted due to health or environmental concerns. These chemicals are subject to the Prior Informed Consent (PIC) procedure, meaning that a country exporting a chemical listed on the Annex must inform and get approval from the importing country to ensure that the chemical is approved for use in the importing country and may enter its borders. This encourages shared responsibility and international cooperation in the management and trade of hazardous chemicals. South Africa is a signatory to this convention, and its execution falls within the mandate of the Department of Forestry, Fisheries and the Environment. For more information on the Rotterdam Convention and the chemicals listed on Annex III, please click here.
- Furthermore, Criterion 4 allows the Registrar to include any additional remedies not falling within the definitions of criteria 1 to 3 posing unmanageable risks to human health or the environment. For example, methomyl is included under this criterion due to frequent wildlife poisonings, paraquat due to its use in suicides and dichlorvos due to illegal sales resulting in accidental poisonings and death. Additional remedies that fall within criterion 4 include formulations of terbufos and methamidophos.
Most of these criteria, especially criteria 1 and 2, relates to products that are acutely toxic (predominantly).
- Acute toxicity refers to serious adverse health effects (i.e. lethal) occurring after a single or short-term oral, dermal or inhalation exposure to a substance or mixture.
- The data on acute toxicity for an agricultural remedy may be based on experimental data or may be calculated according to standardised methods based on the acute toxicity potential of the ingredients in the formulation.
- The test animals used in acute toxicity experiments are mammals (rats and rabbits), used to infer the acute toxicity potential of the chemical on humans.
- Acute toxicity values are expressed as (approximate) LD50 (oral or dermal exposure) or LC50 (inhalation exposure) values or as acute toxicity estimates (ATE; used when the value is calculated). LD = lethal dose; LC = lethal concentration.
- LD50 means the amount of a chemical, given all at once, which causes the death of 50% (one half) of a group of test animals.
- LC50 means the concentration of a chemical in air or of a chemical in water which causes the death of 50 % (one half) of a group of test animals.
- LD50 values are expressed as mg/kg body weight and LC50 values are expressed as mg/L or ppmV.
- The lower the LD50 or LC50 value, the higher the toxicity of the chemical (a lower dose or concentration of the chemical is necessary to cause mortality of one half of the test animals).
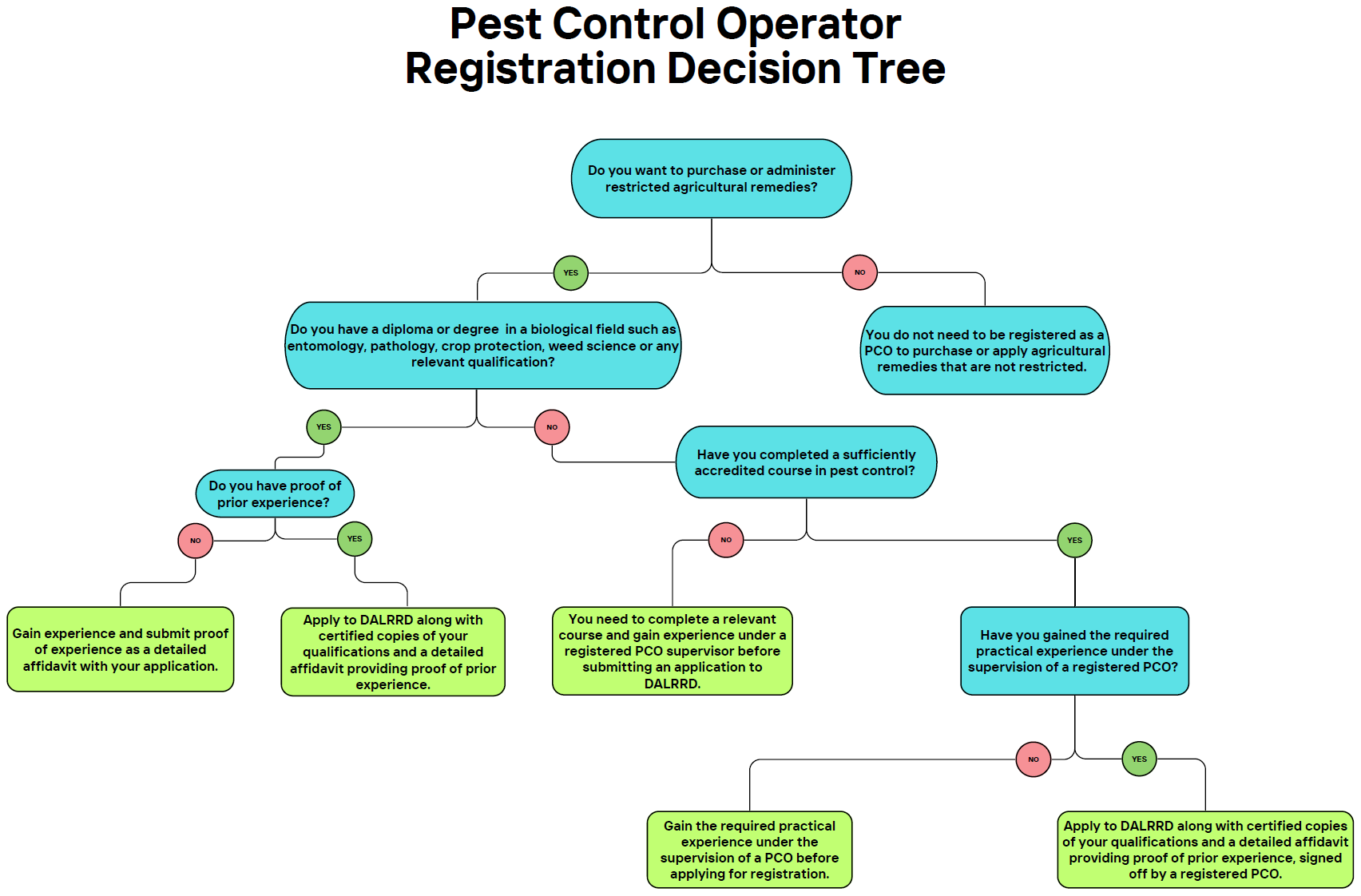
Although “restricted agricultural remedies” was only defined in the “regulations relating to agricultural remedies” in 2023, the requirement for these remedies to be restricted to pest control operators (PCOs) has been included in the “Pest Control Operator regulations” since 2011.
According to the “Pest Control Operator Regulations” of 18 February 2011, although employees who perform pest control for their employer on the employer’s property generally do not need to be registered as pest control operators (PCOs), employees who handle restricted agricultural remedies must be registered as PCOs, or perform such duties under the direct supervision of a registered PCO. This means that a registered PCO must be physically present at the time and place where restricted agricultural remedies are applied. Consequently, in future, farmers that use these remedies will have to ensure that a person working on the farm has the necessary qualifications to oversee the use of these remedies.
The “regulations relating to agricultural remedies” requires that a restriction notice be included on the label of the agricultural remedy to indicate the limitations on use. Although the current regulations state that this notice should be included at the top of the secondary display panel of the label, this has been amended to the top of the main display panel in the revised regulations submitted to the Minister for approval. Thus, a restricted agricultural remedy should be easily identifiable by means of the restriction notice on the front panel of the label (Figure 1). The restrictions will also be communicated under Section 1 of the Safety Data Sheet.
Below is an example of a restriction notice on a restricted agricultural remedy label:
THIS REMEDY IS RESTRICTED DUE TO ACUTE TOXICITY. THIS REMEDY MAY ONLY BE SOLD TO, AND USED BY, A REGISTERED PEST CONTROL OPERATOR, OR BY SOMEONE UNDER THE SUPERVISION OF A REGISTERED PEST CONTROL OPERATOR, AND ONLY FOR THOSE USES COVERED BY THE PEST CONTROL OPERATOR’S SCOPE OF REGISTRATION, AND ONLY AS DIRECTED ON THIS LABEL.
Figure 1: Example of the front panel of a restricted agricultural remedy showing the positioning of the restriction notice.
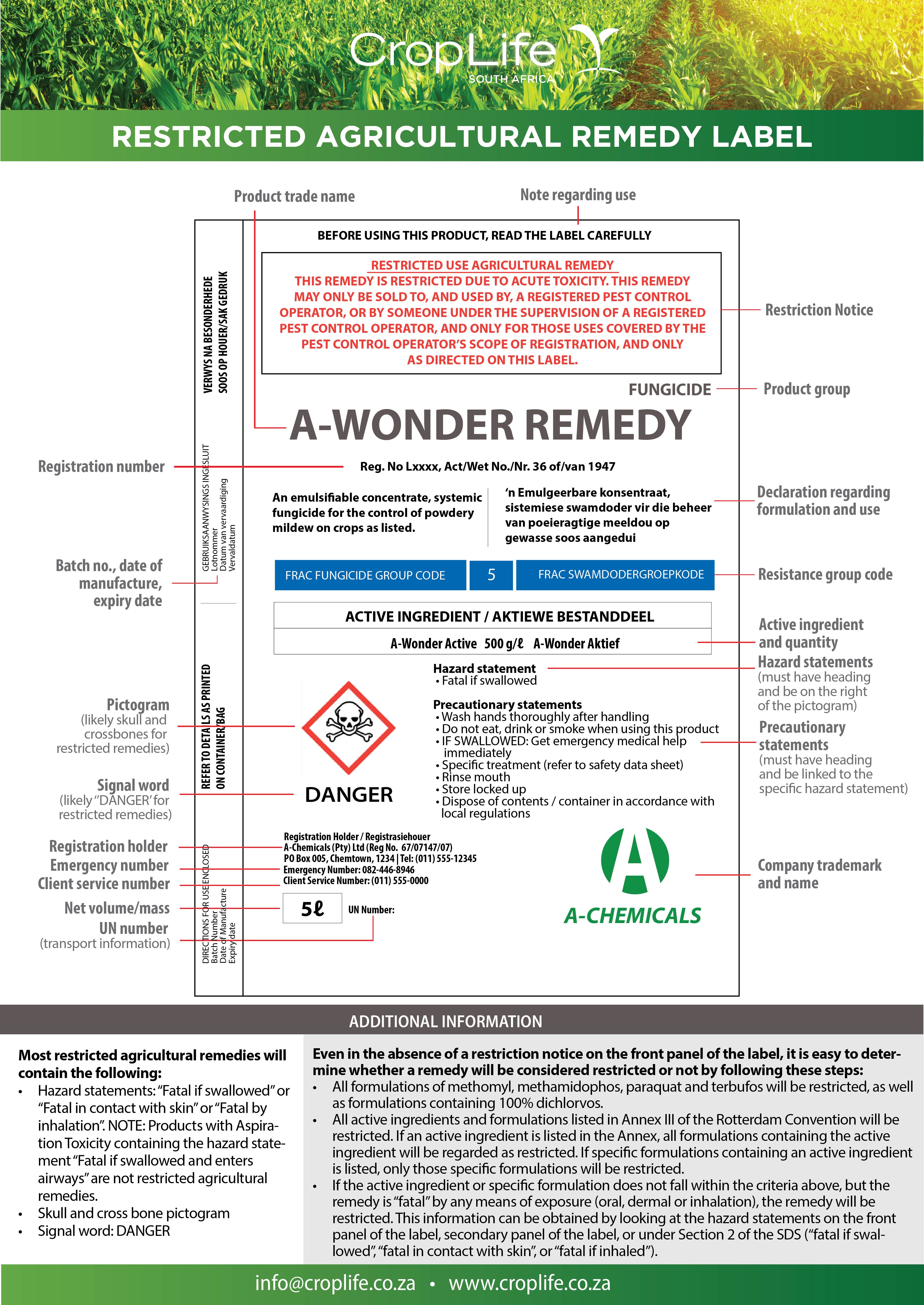
All restricted agricultural remedy labels must contain a similar restriction notice (with some minor variations depending on the reasoning for the restriction) at the top of the main panel of the label.
In addition to the restriction on use of restricted agricultural remedies, sales of these remedies will also be limited. The PCO registration requirement in terms of sales apply to the end-user of the product (farmer/farmworker) only. If a supplier sells a remedy to a distributor/agent who sells the remedy to the end-user, the distributor/agent does not need to be registered as a PCO, only the end-user. Distributors/agents must however ensure that the person they are selling restricted remedies to is registered as a PCO within the applicable field of certification related to the remedy being sold. Thus, if a remedy is being sold for use in agriculture (excluding aerial application or fumigation which has their own fields of certification), the PCO must be certified in the field of “agriculture and forestry” (please refer to the section on Pest Control Operators for more information on the fields of certification).
In accordance with the “Regulations under the hazardous substances Act 15 of 1973”, various measures are already in place for the handling and trading of Group I hazardous substances. Agricultural remedies classified as Group IA hazardous substances include aluminium and zinc phosphide, and those classified as Group IB hazardous substances include all chemicals falling within Class 6.1 (toxic substances) according to the UN Recommendation on the Transport of Dangerous Goods Model Regulations, specified in Appendix E and F of the South African Bureau of Standards Code of Practice 10228. “Restricted agricultural remedies” as defined in the “Regulations relating to agricultural remedies” are predominantly acutely toxic and will thus also be regulated under the “Hazardous Substances Act 15 of 1973”. Therefore, in addition to the requirements already stipulated in the Hazardous Substances Act for Group I hazardous substances, the following will be applicable to “restricted agricultural remedies”:
- A “restricted agricultural remedy” may only be sold to a registered PCO qualified in the specific field of application. Thus, a sale of a restricted remedy may only be made to a person, who upon purchase, provide proof that they are sufficiently qualified and registered to administer the remedy.
- On initial purchase, the registered PCO must provide to the supplier/distributor of the remedy a certified copy of their PCO registration certificate which will be kept on file by the supplier/distributor.
- If the person responsible for sales on a farm is not the person who is registered as a PCO and applying agricultural remedies in the field, it is possible for a designated person who is not registered as a PCO to purchase a restricted agricultural remedy on behalf of a registered PCO. In this case, upon first purchase, the registered PCO will accompany the representative responsible for sales to the supplier/distributor in order for the supplier/distributor to get acquainted with the PCO and sales representative. Along with a certified copy of the PCO registration certificate, the PCO will provide a signed letter to the supplier/distributor giving consent to the sales representative to purchase the restricted remedy on their behalf. These documents will be kept on file by the supplier/distributor.
Pest control operators are also regulated under Act No. 36 of 1947, and a person must apply to the Department of Agriculture, Land Reform and Rural Development (DALRRD) to become registered. Pest control operators are certified in specialised areas of pest control and the following fields of registration are available:
- Fumigation
- Aerial application
- Agriculture and forestry
- Industrial vegetation and noxious weeds
- Landscape
- Supplemental and/or remedial wood treatment
- Structural
- Any other relevant specialisation
Each field necessitates specialised training and certification to ensure that the PCO has the appropriate knowledge and skills to safely and successfully apply the restricted remedy. As a result, a PCO certified to conduct fumigation may not administer structural pest control remedies unless obtaining additional structural pest control certification. Remedies that are registered for use in agriculture (with the exception of aerial application and fumigation), will require a PCO to be certified in the field of “agriculture and forestry”.
The process for registration as a PCO is circulated annually by DALRRD. Below are some of the key requirements:
- A person must do a course to obtain a national certificate in pest control from a relevant accredited academy.
- The person must obtain practical experience under a registered PCO for 6 to 12 months, depending on the relevant field of certification.
- A person with a diploma or degree in a biological field such as entomology, pathology, crop protection, weed science, or any relevant qualification may be exempted from the requirement of practical experience depending on prior experience.
- The person should undergo a medical examination and must submit the template report completed by a qualified medical practitioner with their application.
Following the approval of the PCO application, the certificate will be valid for a period of three years. To retain the certificate, the PCO must submit a renewal application before the validity term expires. It is essential that PCOs provide evidence that they are competent and actively working in the field in which they have obtained the certificate (please refer to Figure 2 for an example of a PCO registration certificate).
CropLife SA circulated on 21 August 2024 a list of the agricultural remedy active ingredients likely to be classified as “restricted”. Since this list was circulated, a few additional active ingredients have been included to the list – please refer to Table 1 below.
This list contains 50 active ingredients currently registered in agricultural remedies in South Africa. Some active ingredients are listed twice due to minor variations in the way the compounds/pesticides were listed in the reference material used to compile the list (for example, paraquat vs paraquat dichloride). To compile this list, only the classification of the active ingredient was taken into consideration, however the classification as a restricted remedy would (in most cases) depend on the classification of the formulation and not just the active ingredient. Consequently, an agricultural remedy containing an active ingredient on the list in low concentrations might not be classified as a restricted agricultural remedy.
Similarly, a remedy might be classified due to the presence of a toxic co-formulant and not the active ingredient, in which case the active ingredient will not be included on the list. Consequently, this list is merely an indication of the remedies that will likely be restricted; however, it should not be regarded as an exhaustive list.
Even in the absence of a restriction notice on the front panel of the label, it is easy to determine whether a remedy will be considered “restricted” or not by following these steps:
- All formulations of methomyl, methamidophos, paraquat and terbufos will be restricted, as well as formulations containing 100% dichlorvos.
- All active ingredients and formulations listed in Annex III of the Rotterdam Convention will be “restricted”. If an active ingredient is listed in the Annex, all formulations containing the active ingredient will be regarded as “restricted”. If specific formulations containing an active ingredient is listed, only those specific formulations will be restricted.
- If the active ingredient or specific formulation is not listed above, but the remedy is “fatal” by any means of exposure (oral, dermal or inhalation), the remedy will be restricted. This information can be obtained by looking at the hazard statements on the front panel of the label, or under Section 2 of the SDS (“fatal if swallowed”, “fatal in contact with skin” or “fatal if inhaled”).
The “regulations relating to agricultural remedies” also defines a class of remedies referred to as “substances of concern”. Most of these remedies are currently being phased out and the Registrar (Act No. 36 of 1947) no longer has the mandate to approve registrations or renew registrations of remedies complying with these criteria, unless the registration holder conducts a risk assessment and apply for a derogation to keep such a remedy on the market for restricted uses. However, this is only possible if the intended use fulfils the derogation criteria provided in the regulations. Although uses of “substances of concern” will be restricted, these remedies are not the same as “restricted agricultural remedies”.
The criteria provided for placing an agricultural remedy in the class of a “restricted agricultural remedy”, is mostly dependent on the remedy being regarded as acutely toxic. “Substances of concern” however are mostly regarded as toxic by chronic exposure (such as the potential of the chemical to cause cancer, induce genetic mutations or interfere with reproduction or the unborn child over long-term exposure). The criteria to consider a remedy as a “substance of concern” and the criteria to consider a remedy as a “restricted agricultural remedy” is not however mutually exclusive and some remedies may fulfil the criteria for both.
Figure 2: Example of a certificate of registration as a Pest Control Operator under Act No. 36 of 1947.

Table 1: List of active ingredients likely to be regarded as “restricted agricultural remedies” according to the “Regulations relating to agricultural remedies” of 25 August 2023. The list only contains active ingredients registered in agricultural remedies in South Africa. The classification of a remedy as “restricted” will, in general, be dependent on the final hazard classification of the formulation, and not just the active ingredient. Consequently, an agricultural remedy containing an active ingredient on the list in low concentrations might not be classified as a restricted agricultural remedy. Similarly, a remedy might be classified due to the presence of a toxic co-formulant and not the active ingredient, in which case the active ingredient will not be included on the list. The list of active ingredients was also cross-checked with the Appendix of SANS 10228 to indicate whether the active ingredient is regulated under the “Hazardous Substances Act” as a Group I hazardous substance.
| Active ingredient | Registered in SA in agricultural remedies | WHO 1A | WHO 1B | GHS Category 1 | GHS Category 2 | Rotterdam Convention | Additional restrictions imposed by Registrar | Regulated under the hazardous substances act |
|---|---|---|---|---|---|---|---|---|
| 1,2-Dibromoethane (EDB) | Yes | X | Yes | |||||
| Abamectin | Yes | X | X | X | Yes | |||
| Alachlor | Yes | X | Yes | |||||
| Aluminium phosphide | Yes | X | X | Yes | ||||
| Azinphos-methyl | Yes | X | X | X | Yes | |||
| Beta-cyfluthrin | Yes | X | Yes | |||||
| Brodifacoum | Yes | X | X | Yes | ||||
| Bromadiolone | Yes | X | X | Yes | ||||
| Bromethalin | Yes | X | X | X | Yes | |||
| Bromoxynil | Yes | X | Yes | |||||
| Cadusafos | Yes | X | X | X | Yes | |||
| Calcium arsenate | Yes | X | Yes | |||||
| Carbofuran | Yes | X | X | X | Yes | |||
| Carbosulfan | Yes | X | Yes | |||||
| Chloropicrin | Yes | X | Yes | |||||
| Chlorothalonil | Yes | X | Yes | |||||
| Coumatetralyl | Yes | X | X | Yes | ||||
| Cyfluthrin | Yes | X | X | Yes | ||||
| DDT | Yes | X | Yes | |||||
| Demeton-S-methyl | Yes | X | Yes | |||||
| Dichlorvos | Yes | X | X | X | Yes | |||
| Difenacoum | Yes | X | X | Yes | ||||
| Difethialone | Yes | X | X | Yes | ||||
| Diquat | Yes | X | Yes | |||||
| Diquat dibromide | Yes | X | Note A | |||||
| Ethoprophos | Yes | X | X | X | Yes | |||
| Fenamiphos | Yes | X | X | Yes | ||||
| Fenbutatin oxide | Yes | X | Yes | |||||
| Fenpropathrin | Yes | X | Yes | |||||
| Fenpyroximate | Yes | X | Note B | |||||
| Fentin hydroxide | Yes | X | Yes | |||||
| Flocoumafen | Yes | X | X | X | Yes | |||
| Formetanate | Yes | X | X | Yes | ||||
| Lambda-cyhalothrin | Yes | X | Yes | |||||
| Magnesium phosphide | Yes | X | X | Yes | ||||
| Methamidophos (All formulations) | Yes | X | X | X | X | Yes | ||
| Methidathion | Yes | X | X | Yes | ||||
| Methiocarb | Yes | X | Yes | |||||
| Methomyl | Yes | X | X | X | Yes | |||
| Mevinphos | Yes | X | X | X | Yes | |||
| Omethoate | Yes | X | Yes | |||||
| Oxamyl | Yes | X | X | Yes | ||||
| Paraquat | Yes | X | Yes | |||||
| Paraquat dichloride | Yes | X | X | Yes | ||||
| Parathion (All formulations) | Yes | X | X | X | X | Note C | ||
| Parathion-methyl | Yes | X | X | Yes | ||||
| Prothiofos | Yes | X | Yes | |||||
| Tefluthrin | Yes | X | X | X | Yes | |||
| Terbufos | Yes | X | X | X | X | X | Yes | |
| Zinc phosphide | Yes | X | X | Yes |
Note A: Diquat is regulated as a Group IB hazardous substance which should encompass diquat dibromide.
Note B: Active ingredient flagged only for acute toxicity by inhalation which was not previously covered by the WHO classification scheme.
Note C: Paraquat is regulated as a Group IB hazardous substance which should encompass paraquat dichloride
![]() Click here to refer to our FAQ if you have queries that might not be covered in the above sections.
Click here to refer to our FAQ if you have queries that might not be covered in the above sections.
 PDF Document
PDF Document